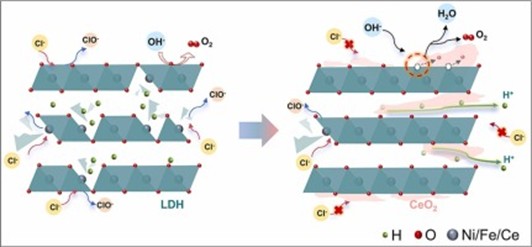
Industrial water electrolysis typically occurs at temperatures higher than ambient. The anodic oxygen evolution reaction (OER) in water electrolysis is inherently temperature-dependent, with enhancements in thermodynamics and kinetics at higher temperatures. However, it is often overlooked that material properties at elevated temperatures can differ significantly from those at room temperature. In this study, NiFe-based layered double hydroxide (LDH)/CeO2 is utilized as anodic electrocatalyst for both ambient- and elevated-temperature water/seawater electrolysis. The optimal catalyst displays favorable oxygen ion conductivity and thus enhanced OER activity. The partial coverage of LDH with CeO2 effectively mitigates direct chlorine adsorption. Additionally, its high proton conductivity prevents proton accumulation within LDH interlayers and ensures stability. Notably, the enhanced mixed ionic conductivities at elevated temperature contribute to significant improvements in OER performance. This study underscores the effectiveness of employing mixed ionic conductors for OER and highlights the importance of characterizing OER process under elevated temperature for practical applications.